Patient Informed Consent: MRI-Guided Laser Focal Therapy Treatment for Prostate Cancer
Please watch this consent video carefully and completely in order to prepare yourself, family members, and caregivers for the procedure. Do not skip ahead. It is essential that you watch it in its entirety. We want you to be as prepared as possible. This video describes the possible risks connected with performing laser focal therapy for prostate cancer. Should you have any questions or concerns, we strongly encourage you to contact our office prior to your procedure so that we may clarify any pertinent issues. “An educated patient is the best patient.”
To contact the HALO Diagnostics office and procedure location by phone, call (760) 776-8989
We are here to support you, please contact us if you have any questions or need assistance of any kind.
General Procedure Risks
Purpose
The purpose of this is to utilize MR (magnetic resonance) guided laser focal therapy to ablate prostate cancer lesions. MR uses large magnets and radio waves to generate images of organs in the body. The laser uses light to heat a target area to destroy cancerous cells.
The laser systems that will be used have been used for the treatment of brain, bone (spine), thyroid, and liver cancers.
The laser applicator placement will be performed using the Invivo DynaLOC software and DynaTRIM trans-rectal biopsy guidance system. This system is cleared (approved) by the U.S. Food and Drug Administration (FDA) for such uses.
Definition
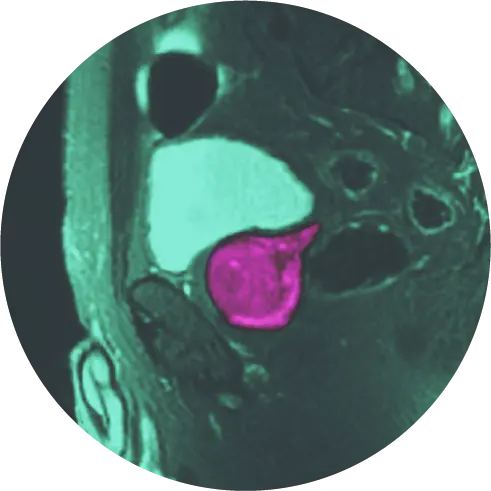
Laser focal therapy of the prostate gland is a minimally invasive alternative to the standard treatment of prostate cancer. It is a targeted procedure that focuses on a single area of the tumor as opposed to whole-gland therapy. For the procedure, a laser applicator is introduced under MRI guidance via the rectum. Once in the gland, a nontherapeutic “test” dose is administered to verify proper placement in the target, then increased while “real-time” temperature change is displayed on a thermal map.
Once the entire area is treated (approximately 2 minutes), the laser is turned off. The number of treatments depends on the size, number, location, and shape of the target lesion(s).
Benefits
- <1% risk of erectile dysfunction, risk of incontinence, and risk of infection
- Ambulatory outpatient procedure lasting 4 hours
- Rapid recovery: Most patients can return to work the next day
- No general anesthesia required
- Highly accurate (within 1 millimeter)
- Does not limit the option to treat with radiation therapy or surgery if needed later
Preparation
Prior to the procedure, a medication review will be scheduled with your clinician. Any anticoagulant medications must be stopped prior to the procedure as ordered by your treating physician. The procedure cannot be scheduled if you are currently on or have recently taken any anticoagulant medications (“blood thinners”) that may interfere with your ability to clot your blood. The most common of these medications are anticoagulants such as coumadin, Xarelto, Pradaxa, and over-the-counter medications also known as nonsteroidal anti-inflammatory drugs such as Aspirin, Celebrex, Motrin, Ibuprofen, Advil, Aleve, and other pain reliever compounds (whether prescription or over the counter). Review the list of drugs you take carefully as many over-the-counter medications like Alka-Seltzer and Pepto Bismol contain Aspirin. If in doubt check with your clinician. If needed, use Tylenol (acetaminophen) instead.
Prior to the procedure, you must fast for 8 hours. Do not eat any food or consume any liquids during this time. We want to avoid gas or stool in the rectum for your procedure. Upon arrival, you will be provided an enema. Depending on the treatment location, you may also have a urinary catheter inserted prior to your procedure.
You may be given an oral antibiotic which should be taken as directed by our physicians. Most patients will simply receive intravenous antibiotics at the time of the procedure.
You must have a friend or family member come with you to your appointment to drive you home and remain with you overnight. This is extremely important since you will receive conscious sedation. Plan to remain in the area for at least two days after your procedure for observation. If you cannot bring a friend or family member there are local services that can provide you with overnight care the day of your procedure.
Procedures
Baseline Evaluation Procedures
Prior to laser focal therapy, the following study procedures will be performed.
- Medical history and quality-of-life questionnaires.
- Blood tests – including but not limited to PSA.
- Multiparametric MRI of the prostate – including MRI with contrast (gadolinium).
- Estimate of prostate gland volume.
- Biopsy (a targeted biopsy should be performed and possibly a systematic biopsy).
Treatment Procedures
The actual procedure typically takes up to 4 hours. You will have a local anesthetic and be given an IV sedative medication before the procedure. You will also receive a regional anesthetic which is injected into both sides of the prostate gland. You will get a urinary catheter placed before your procedure if the tumor is down low in the apex, or afterward to preserve urine flow as the prostate can swell from inflammation. You’ll be placed lying face down on the MRI table. A laser guide will be gently placed in your rectum. Although it is slightly uncomfortable, very few patients believe it is painful. After confirming the precise position of the small laser applicator with MRI, we will then treat the target area(s). The amount of time the laser is on will depend on the decision of the surgeon, your anatomy, and possibly on whether you have had a therapy done in the past.
At the end of the laser treatment, post-treatment MR imaging will be performed.
Post Procedure
After the procedure, you might feel a bit sore in the rectal or anal area for a few hours. Patients with hemorrhoids might have discomfort a bit longer. It is very common to see some blood from the rectum, on the stool with the next bowel movement, or on the toilet paper especially that day and rarely the next day. Again, this is more common in patients with hemorrhoids. A small amount of blood in the urine or some discoloration of the urine is also a possibility. You may commonly see blood in your semen (ejaculation) for 1-2 days and sometimes up to 6 weeks. It might be red or just discolor your semen brown.
You are restricted from driving immediately following the treatment. You have no other restrictions after the procedure other than to take it easy that day and not engage in strenuous activity for 4-6 weeks. Have a family member or friend drive you home following the procedure. This is temporary and the duration will depend on the size and location of the treatment(s).
Post-treatment Evaluation
It is recommended that you follow up approximately 5-14 days, 6 months, and 12 months after treatment and as indicated thereafter at our HALO center or any other radiology center. At each follow-up visit, you should undergo the following exams where your insurance will be charged:
- History including recent PSA. (Please mail in at 3 mos.)
- Quality-of-life questionnaire scores evaluation. (Please mail in at 3 mos.)
- Assessment of any side effects or problems.
- mp-MRI of the prostate – including MRI with contrast (gadolinium).
- Biopsy (a targeted biopsy will be performed and possibly a systematic biopsy).
At your first post-treatment follow-up visit, you should receive a pelvic ultrasound to evaluate your bladder function.
Additionally, at approximately 6 months post-treatment, you should undergo multiparametric MRI and MR- guided biopsy if indicated. Biopsy samples, if performed, will be sent for evaluation by a pathologist. At one year you should have mp-MRI and surveys. We will need your PSA at each follow-up interval. This is a very important detail.
After approximately one year, you should undergo a multiparametric MRI and submit surveys and questionnaires.
Possible Complications of the Procedure
This procedure, regardless of complexity or time, may be associated with unforeseen problems. Problems may be immediate or even quite delayed in presentation. While we have discussed these and possibly others in your consultation, we would like you to have a list so that you may ask questions if you are still concerned. Aside from anesthesia complications, it is important that every patient be made aware of all possible outcomes which may include, but are not limited to:
- Pain and/or discomfort: You may feel sensations of discomfort or heat after your procedure. Discomfort is rare and may be managed with acetaminophen.
- Excessive Bleeding from the Rectum/Anus: It is uncommon to require any treatment and the majority of the time bleeding stops on its own. This is far more common in patients with hemorrhoids or arterial bleeding. In the unusual circumstance that excessive bleeding occurs or a severe infection occurs this may require emergency room care and possible hospitalization.
- Infertility: By the time prostate cancer has been diagnosed and requires treatment, fertility status following treatment has become essentially irrelevant as reproductive issues have already been settled by most men. However, for some men who wish to consider fathering more children, fertility status following treatment for prostate cancer may be significant and relevant.
For those patients, it is important to note that while focal laser therapy may not substantially affect fertility (i.e. prostate cancer lesions located anteriorly within the prostate) making focal laser therapy in patients who wish to preserve fertility preferable compared with other treatment options which are less precise and therefore may compromise fertility, there are, nonetheless, certain situations where prostate cancer focal laser therapy may result in the destruction of one or both ejaculatory ducts which deliver semen for ejaculation and thus significantly reduce or entirely eliminate fertility.
Additionally, focal laser therapy of a prostate cancer lesion may result in retrograde ejaculation (ejaculation into the bladder rather than into the urethra for expulsion) resulting in infertility. Therefore, for those men who may wish to father children following focal laser therapy, we recommend banking sperm PRIOR to focal laser therapy.
- Blood Clots in the Urine: The device can enter the middle of the prostate where the urethra or the neck of the bladder are located and cause blood in the urine. If the bleeding is significant, it can cause clots that can block the urine flow. A urinary catheter will be inserted to avoid this problem.
- It has been our experience that while the catheter might be uncomfortable it helps to avoid possible issues.
- Urinary Retention: Even in the absence of bleeding, the prostate can become swollen from the treatment or secondary to infection.
This is another reason why post-procedure urinary catheter placement is important. Usually, the problem resolves with time after the swelling goes down. Patients at greater risk are those who already have difficulty urinating before the procedure due to BPH (Benign Prostatic Hyperplasia).
- Urinary Tract Infection or Urosepsis: Although you will receive IV antibiotics, it is possible for you to get an infection.
It might be a simple bladder infection that presents with symptoms of burning urination, urinary frequency, and a strong urge to urinate. This will usually resolve in a few days with antibiotics. If the infection enters the bloodstream, you may feel very ill. This type of infection often presents with urinary symptoms and any combination of the following: fevers, shaking, chills, weakness or dizziness, nausea, and vomiting. You may need a short hospitalization for intravenous antibiotics, fluids, and observation. This is more common in diabetics, patients on long-term steroids, or patients with any disorder of the immune system. Lastly, an abscess of the prostate, while quite rare, can develop. This is an infection cavity that may respond to antibiotics alone or need surgical (needle) drainage. It can begin with urinary symptoms but also progress to the symptoms of bloodstream infection. Urinary retention is also possible with an abscess.
- Erectile Dysfunction (ED): Inability to achieve or maintain an erection can occur following this procedure in less than 1% of patients. This condition may be temporary or permanent. A decrease in the volume of seminal fluid is also a possibility. This may result in infertility.
- Urinary Incontinence: The ability to voluntarily control urination may be lost in less than 1% of men undergoing this procedure. This may be a temporary or permanent condition.
- Tingling/Numbness of the Penis or Scrotal Pain: Some men may feel an urge to urinate during the procedure. Some feel tingling in their penis or scrotum due to referred pain. Similar to when you hit your funny bone, your fingers may tingle. These sensations generally stop after each 2-minute treatment, and rarely persist after the procedure. These symptoms are self-limiting.
- Scrotal pain has also been reported following the procedure. Our multilayered anesthetic techniques help to manage this.
- Rectal Fistula: Rarely an abnormal connection between the rectum and the urethra or urinary bladder may develop. This may require surgery for treatment. Our team devised a method to place safety boundaries over important structures like the rectal wall to avoid this serious problem.
- Residual Prostate Cancer: Several studies have shown that there is improved survival after ten years following whole-gland definitive therapy (radical prostatectomy, radiation therapy) compared with active surveillance (watchful waiting) for the treatment of prostate cancer confined to the prostate gland. Laser focal therapy has similar rates of recurrence as prostatectomy and radiation without the side effects of incontinence and impotence.
- Thermal injury to nearby organs: Injuries due to the heat produced by the laser have not been seen in patients to date;
- However, there is a possibility that heat damage could occur if the laser fiber has a crack along its length. We prevent this by visually inspecting every fiber prior to its use to ensure energy is being emitted only from the tip.
- Carbonization of laser applicator: This may result in a portion of the applicator being retained inside the prostate or being expelled.
The gadolinium contrast agent used with MRIs has known risks. The most common are:
- strange tastes;
- changes in smells;
- injection site reactions;
- nausea;
- generalized feeling of warmth; headache; and
- dizziness.
Rarely, serious, life-threatening allergic-type reactions can occur.
If you have symptoms of any of the above, especially those of infection, you must contact HALO Diagnostics immediately or go to the nearest Urgent Care or emergency room.
Alternatives
Your alternatives to this procedure include:
- Active Surveillance.
- Radical Prostatectomy.
- Radiation Therapy.
- Brachytherapy.
- Androgen Deprivation Therapy.
- Another experimental treatment/procedure.
The doctor will discuss these alternatives with you.
Confidentiality
Your information may also be given to the U.S. Food and Drug Administration (FDA). It may be given to similar governmental agencies in other countries.
Medical records which identify you and the consent form signed by you may also be looked at and/or copied for research or regulatory purposes by:
- Department of Health and Human Services (DHHS) agencies,
- the institution where the research is being done,
- the California Cancer Registry, and
Although absolute confidentiality cannot be guaranteed because of the need to give information that identifies you to these parties, we make every effort to anonymize information for all of our patients.
Conclusion
Although currently not considered standard of care for treatment of prostate cancer, Phase II investigational studies have demonstrated treatment efficacy comparable to radical prostatectomy and possibly superior to radiation therapy regarding prostate cancer recurrence rates with superior quality of life metrics (i.e. erectile dysfunction, urinary incontinence, rectal injury) allowing Laser Focal Therapy to move from investigational to translational on the status and ultimately standard of care.
Whom to Contact
For answers to questions relating to this procedure, or to report a concern, complaint, or injury, or for information regarding laser procedures you may contact:
HALO Diagnostics office: 760-776-8989
Bernadette M. Greenwood MSc., RT(R) (MR) (ARRT):
760-776-8989 x747 (24 hours)
262-269-8764 (24 hours)
If you are experiencing any medical emergency, dial 911 or go to the emergency room or urgent care.
Once I sign the physical Informed Consent Document, I am agreeing to undergo magnetic resonance image-guided transrectally delivered laser-induced interstitial thermal therapy for laser focal therapy of prostate cancer in an outpatient setting.